即将举行的网络研讨会:实现无需离心的流式细胞术样品制备工作流程的标准化
Developing neoantigen-based cancer vaccines necessitates comprehensive cellular characterization for iterative therapeutic improvement, which Laminar Wash™ systems may facilitate at multiple steps.
Vaccines are probably the second-most effective advancement in the annals of human medicine, after sanitation. Most of us recognize the value of vaccines in infectious disease. Yet, swept under the rug amongst the COVID-19 global pandemic is another form of immune-boosting injection combating a terrible disease: cancer vaccines. These therapies represent true “personalized medicine;” an individual’s tumor-specific epitopes are measured, manufactured, and presented back to the patient’s immune system to mount a response, often in combination with other therapies like immune checkpoint inhibitors (ICIs).
Delivering on the promise
Therapeutic cancer vaccines work similarly to those against infectious diseases, whereby recognizable molecular signatures (epitopes) are presented to the immune system, which mounts a defense and clears affected cells. Only this time, the epitopes are specific to an individual’s tumor. While the idea of vaccines against individual cancers is not new, clinical trials have recently progressed due to a convalescence of advanced sequencing and cell analysis methods, neoantigen epitome prediction algorithms, and a better understanding of immune cell biology. In a recent review article published in Nature Reviews Clinical Oncology, Eryn Blass and Patrick Ott of the Dana-Farber Cancer Institute paint a fantastic overview of the process, progress, and problems facing these tantalizing therapies, specifically those directed towards neoantigens.
Despite the tsunami of “better, faster, cheaper” sequencing and computational analysis supporting their development, therapeutic cancer vaccines still face hurdles related to rapid turnaround time and costs associated with identifying a patient’s epitope, scaling up a biologic and delivery mechanism, and reintroducing back to the patient. Modern diagnostic tools also support the case for the continuous monitoring of patients’ key immune- and tumor-related biomarkers to ensure the right cells are persistently recognizing and infiltrating the tumor. Finally, such efforts fill in immune knowledge gaps that may inform on better therapies, such as elucidating the roles of tissue-resident memory T cells and peripheral memory T cells.
What does this mean for researchers, entrepreneurs, and clinicians eager to deliver on the promise of personalized, neoantigen-directed vaccines? Collecting heaps of samples for analysis—peripheral blood, tumor biopsies, and not to mention the preclinical models supporting these new modalities. “Importantly, detailed assessments of the characteristics of the immune responses induced by vaccination could be used to inform iterative improvements in the approach to vaccine design and administration,” reads the caption of Bloss and Ott’s Figure 4 describing a proposed workflow for vaccine development.
Streamlining sample preparation and improving data readout
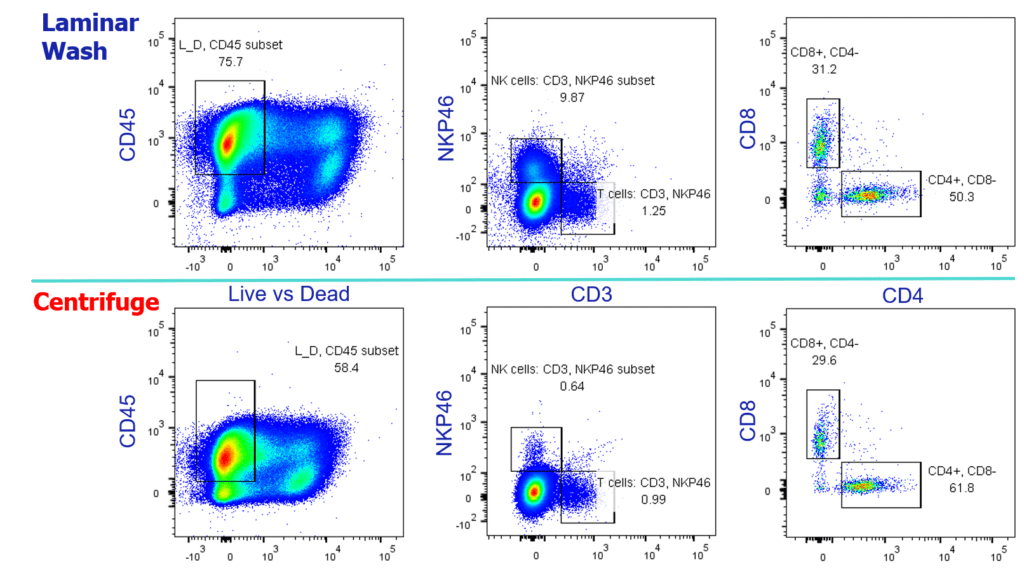
Cellular and molecular characterization methods—from human leukocyte antigen (HLA) typing to multi-color flow cytometry to single-cell RNA sequencing—demand clean samples prepared reproducibly and consistently to ensure immune cells of interest are mounting robust responses to administered neoantigens or lysate-exposed dendric cells. Removing variation across sample collection times and sites becomes critical—a clear need for sample prep automation. Routine monitoring of actively infiltrating (and hopefully not exhausted) T cells, such as CD8+ and CD4+ cells, as well as other important players like dendritic cells and natural killer cells can be tricky, due to the rarity of these subpopulations and low cell numbers present in the tumor microenvironment.
For such a time-sensitive and resource-demanding enterprise like cancer vaccine development, taking advantage of Laminar Wash technology can significantly streamline sample processing and yield superior data quality. By bypassing the centrifuge, Laminar Wash systems remove the guesswork and inconsistencies from cell washing and retain higher levels of target immune cells. In the scope of cancer vaccines, overall survival and resistance to combination ICI therapy can hinge on the abundance and diversity of tumor-infiltrating CD8+ and CD4+ T cells, respectively (the latter revealed in a single-cell transcriptomics study). As our collaborator at Charles River Laboratories, Dr. Christoph Eberle presented in our webinar, Laminar Wash outperforms conventional methods in retaining viable tumor-infiltrating leukocytes. Rely on the centrifuge to measure these crucial biomarkers at your own risk (see figure).
As Blass and Ott specify in their workflow figure, autologous cancer vaccine development is an iterative process necessitating multiple sample collections to monitor immunogenic success in the patient. Laminar Wash may be deployed to facilitate several indicated steps, including HLA typing, vaccine platform optimization, and immune assessment by immunophenotyping and single-cell sequencing. A next-generation therapy like neoantigen cancer vaccines deserves a next-generation sample prep technology like Laminar Wash.



